LA JOLLA, CA—At this moment, the world has few tools to combat deadly filoviruses, such as Ebola and Marburg viruses. The only approved vaccine and antibody treatments protect against just one filovirus species.
Scientists at La Jolla Institute for Immunology (LJI) are working to guide the development of new antivirals by leading some clever enemy reconnaissance. These researchers use high-resolution imaging techniques to examine a virus’s molecular structure—and uncover where a virus is vulnerable to new therapies.
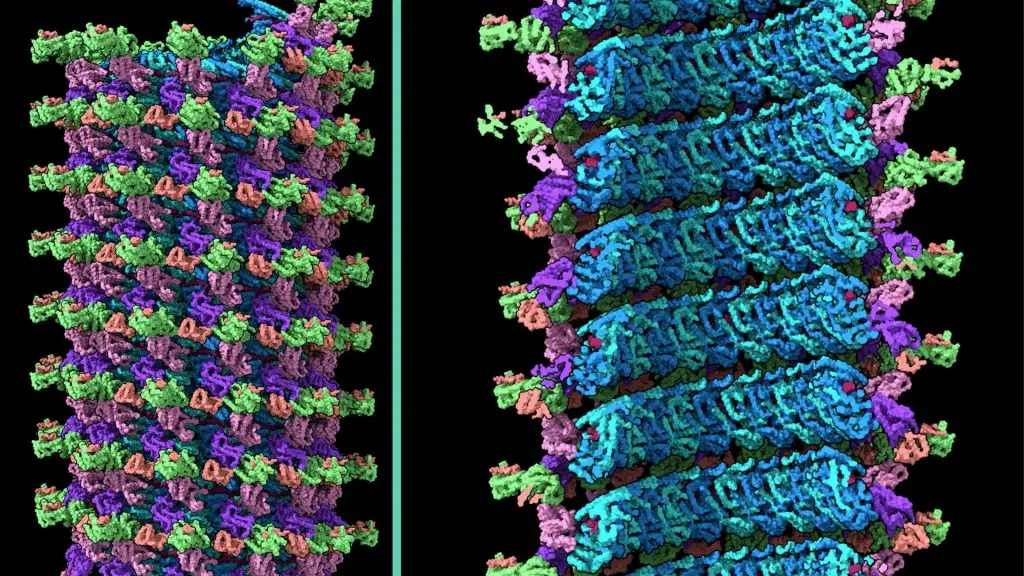
In a new Cell study, scientists in LJI’s Center for Vaccine Innovation share the first detailed, complete images of a viral structure called the Ebola virus nucleocapsid. This breakthrough may accelerate the development of antivirals that target this viral structure to combat several filoviruses at once.
“A universal antiviral is the dream for stopping any kind of viral disease,” says LJI Staff Scientist Reika Watanabe, Ph.D. who led the study as a first author. “This study brings us a step closer to finding a universal antiviral.”
Inspecting the enemy
Ebola virus relies on its nucleocapsid structure to protect and replicate its own genetic material inside host cells—and suppress host cellular immunity. Thanks to the nucleocapsid, Ebola can turn infected host cells into virus-making factories.
For the study, Watanabe achieved a first in science—by harnessing an imaging technique called cryo-electron tomography, she glimpsed Ebola’s nucleocapsid structure at work inside actual infected cells.
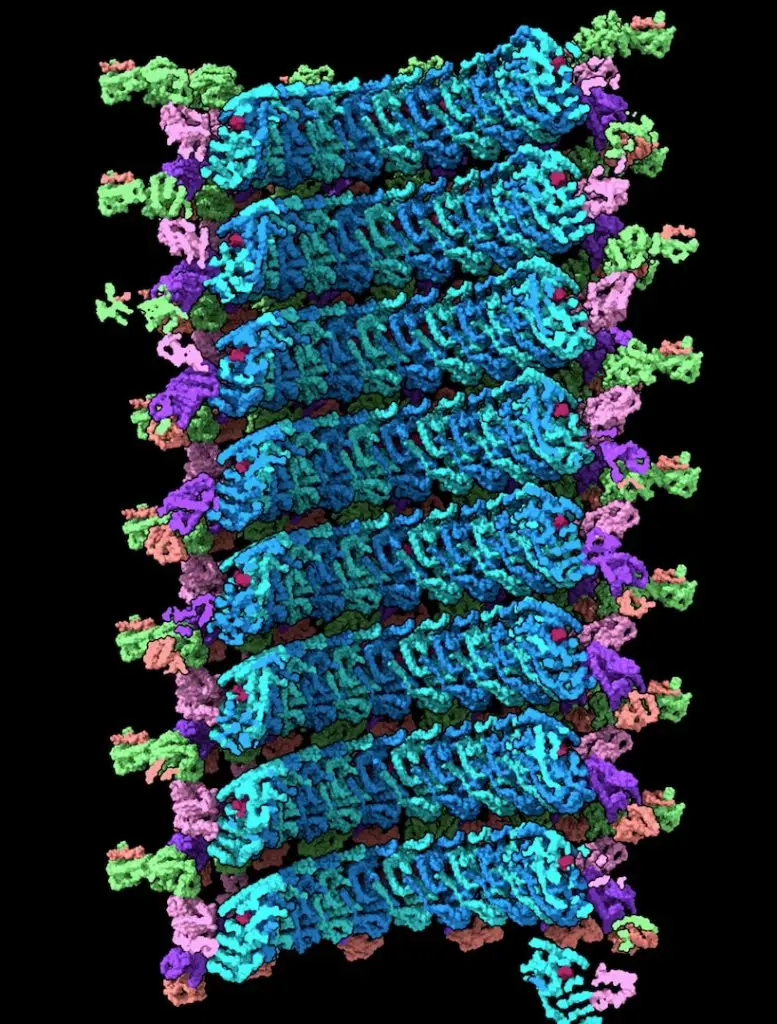
At first glance, the Ebola virus nucleocapsid looks like a coiled telephone cord. Watanabe revealed the stages of coiling and compression of the coil. She also discovered that the nucleocapsid’s cylindrical shape is made up of three layers. Each layer plays a different role as the virus replicates in host cells. Before LJI’s imaging studies, the existence of the outer layer was entirely unknown.
Watanabe’s work also shows how this outer layer is composed, and how it provides a flexible tether between the nucleocapsid and the viral membrane.
“We found that the core protein adopts different forms in the distinct layers of the nucleocapsid to play different roles,” says LJI Professor, President & CEO Erica Ollmann Saphire, Ph.D., MBA, who served as study senior author.
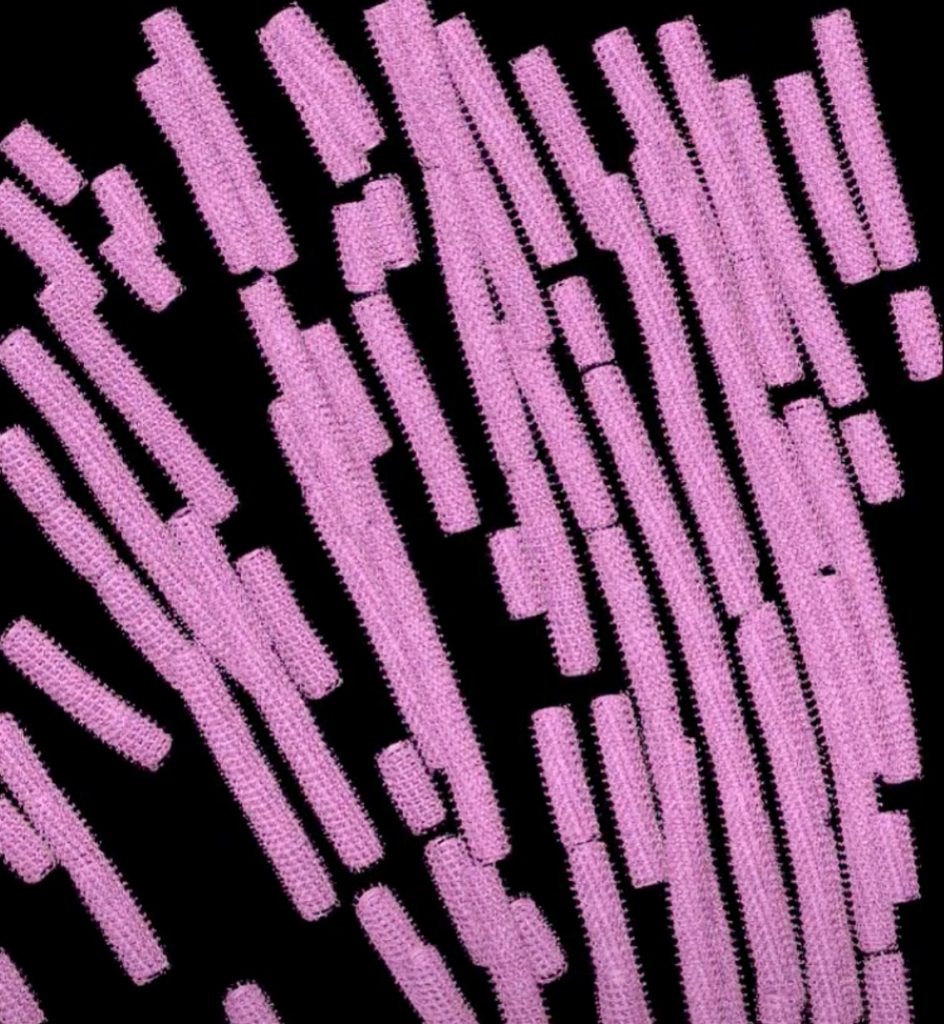
Watanabe’s further investigations revealed how the proteins in these layers make contact with each other during assembly in host cells—and how the Ebola virus rearranges these proteins when nucleocapsids help form new viral particles.
“This study solves several puzzles in the field,” says Saphire.
Targeting Ebola virus—and more
As Watanabe explains, targeting the nucleocapsid spells game over for the virus. “If you don’t have a nucleocapsid, nothing can happen. That’s the core of the virus,” she says.
In fact, the Ebola virus nucleocapsid plays such a critical role in infection that Watanabe suspects the nucleocapsid’s overall structure may have stayed the same as filoviruses evolved. Scientists call this kind of crucial structural feature “conserved” when it is shared across related species.
Indeed, all pathogenic filovirus species known so far, including Ebola and Marburg virus, share a conserved nucleocapsid structure, says Watanabe. She’s now leading further research to study nucleocapsid assembly more closely in Marburg virus.
Additional authors of the study, “Intracellular Ebola Virus nucleocapsid assembly revealed by in situ cryo-electron tomography,” include Dawid Zyla, Diptiben Parekh, Connor Hong, Ying Jones, Sharon L. Schendel, Willian Wan, and Guillaume Castillon.
The study was supported by the National Institutes of Health (NIH, grant R21AI180801).
DOI: 10.1016/j.cell.2024.08.044
###





