LA JOLLA, CA—Scientists at La Jolla Institute for Immunology (LJI) are investigating how the immune system’s T cells react to a wide variety of coronaviruses, ranging from SARS to common cold coronaviruses. Their goal is to guide the development of vaccines that could halt future pandemic by combatting many types of coronaviruses at once.
“While it was recognized that coronaviruses were potentially dangerous viruses, because of SARSCoV and MERS viruses causing very severe disease in humans, nobody knew that the next pandemic was going to be caused by SARS-CoV-2,” says LJI Professor Alessandro Sette, Dr.Biol.Sci. “So the issue now is how can we develop strategies that are broadly applicable to different viral families of concern?”
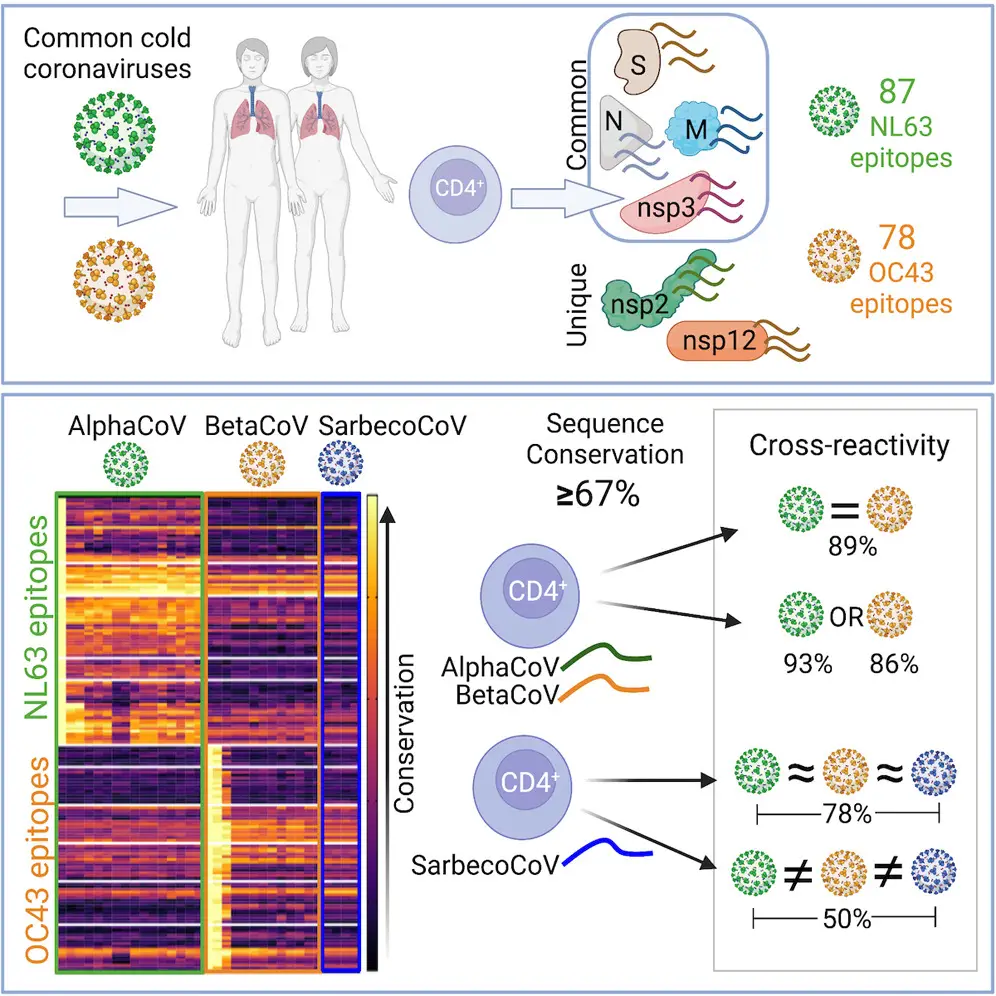
In their latest collaboration, published in Cell Reports Medicine, Sette and LJI Research Assistant Professor Alba Grifoni, Ph.D., show that T cells can recognize several different viral targets, called “antigens,” shared between most coronaviruses, including common cold coronaviruses and SARS-CoV-2. They also looked more in-depth at what fragments of these antigens, called “epitopes,” are recognized and how conserved they are across different coronaviruses.
T cells can recognize several different viral targets, called “antigens,” shared between most coronaviruses, including common cold coronaviruses and SARS-CoV-2. (from Tarke et al., Cell Reports Medicine, 2023)
“This study suggests a way to enhance vaccines so that they have broader activity against many different coronaviruses and variants,” says Grifoni.
What coronaviruses have in common
Sette and Grifoni are experts in studying which of the antigens that make up a virus’s structure are recognized by T cells down to the epitope level. While viruses with similar protein sequences tend to be closely related, even more distant viruses can have some smaller sequences in common. If sequences are recognized by T cells, this immune response can recognize multiple viruses from the same family, even if the viruses themselves are not as similar.
The body’s memory CD4+ “helper” T cells patrol the body for protein sequences belonging to past viral invaders. These T cells help launch the immune system attack against a repeat offender—or any closely related pathogens—they come across. This kind of “cross reactivity” is exactly what scientists want to harness to train immune cells to fight many types of coronaviruses at once.
No one had been exposed to SARS-CoV-2 before 2019. Still, humanity was no stranger to coronaviruses. Some coronaviruses cause common colds, others have been shown to cause severe respiratory disease. Of the two groups of coronaviruses, alpha and beta, scientists have so far found beta coronaviruses to be most likely to have pandemic potential and cause severe disease based on three different outbreaks. The 2002–2003 SARS outbreak, the 2012 MERS outbreak, and more recently SARS-CoV-2, were all caused by beta coronaviruses. Alpha and beta coronaviruses also share similarities in some of the epitopes recognized, and previous research from Sette and Grifoni has demonstrated that T cells against common cold coronaviruses can “cross-react” with SARS-CoV-2.
Taking aim at four viral targets
For the new study, the goal was to see exactly which viral protein sequences or epitopes prompted the strongest reactions from T cells cross reactive across different coronavirus types.
The researchers first comprehensively analyzed T cells collected from 88 patients before the COVID-19 pandemic. These patients had never been exposed to SARS-CoV-2, of course, but they had been exposed to other types of common cold coronaviruses belonging to either the alpha or beta groups. Researchers used these samples to define which viral antigens and which specific epitopes were recognized by T cells.
Then the LJI team, in collaboration with Professor Richard Scheuerman, Ph.D., of the J. Craig Venter Institute, applied a computational approach to predict which epitopes might be the same between different coronaviruses including SARS-CoV-2. This work, led by LJI Postdoctoral Fellow Alison Tarke, Ph.D., revealed 18 coronavirus epitopes highly conserved across multiple coronaviruses, suggesting these epitopes could induce cross-reactive T cells.
The LJI researchers showed that T cells against alpha or beta common cold coronaviruses tend to be cross-reactive across the two different groups. These coronaviruses had a lot of similarities in their epitope sequences, and T cells showed cross-reactivity in 89 percent of tests.
Cross-reactivity declined to 50 percent when the T cells encountered SARS-CoV-2. This means that although SARS-CoV-2 resembles a distant relative of common cold coronaviruses, it still shares protein sequences with members of its family.
Future coronavirus vaccines could leverage a combined approach using antibody target and T cell responses against epitope sequences conserved across the many types of coronaviruses. “The key finding here is that we could potentially develop vaccines that would focus immune responses on these shared sequences, allowing recognition of many different viruses at once,” says Grifoni. “While the SARS-CoV-2 Spike protein is the major target for antibodies, T cells can recognize additional antigens that are conserved across different coronaviruses. The combination of the two could be optimal in the design of a panCorona vaccine.”
“This work is also exciting because it suggests this is a viable strategy to induce other families of viruses of concern in light of potential future pandemics, such as for example the family of viruses causing influenza, or the ones causing hemorrhagic fevers, or the family of viruses which includes dengue and Zika virus,” adds Sette.
Additional authors of the study, “Targets and cross-reactivity of human T cell recognition of Common Cold Coronaviruses,” were Yun Zhang, Nils Methot, Tara M. Narowski, Elizabeth Phillips, Simon Mallal, April Frazier, Gilberto Filaci, Daniela Weiskopf, Jennifer M. Dan, Lakshmanane Premkumar and Richard H. Scheuermann.
This study was supported by the National Institutes of Health’s National Institute of Allergy and Infectious Diseases (P01 AI168347, Contract No. 75N93019C00065, HHS75N93019C00076) and a PhD student fellowship through the Clinical and Experimental Immunology Course at the University of Genoa, Italy.
DOI: 10.1016/j.xcrm.2023.101088
###





