Our long-term research focus has been to understand how signaling pathways control gene expression, using T cells and other cells of the immune system as models. In collaboration with the lab of Professor Patrick Hogan, we previously showed that gene expression is regulated by calcium influx into many different types of cells: it involves a process known as store-operated calcium entry, which activates a phosphatase, calcineurin, which dephosphorylates and sends a transcription factor, NFAT, to the nucleus [https://pubmed.ncbi.nlm.nih.gov/12975316/]. NFAT turns on a large number of genes, in a manner appropriate to the cell type and mode of stimulation; in T cells, it controls both the positive transcriptional programs of T cell “activation” and negative programs, known variously as “exhaustion”, “anergy” or “dysfunction”, that attenuate T cell activation [https://pubmed.ncbi.nlm.nih.gov/25680272/]. These terms all refer to a hyporesponsive state observed under conditions of sustained antigen stimulation in cancer and chronic viral infections.
To understand the biological implications of this hyporesponsive program, our lab uses mouse models of anti-tumor responses that involve adoptive transfer of T cells bearing chimeric antigen receptors (CAR-T cells). Using these models, we have identified important targets of NFAT in the exhaustion program, including transcription factors of the NR4A and TOX families [https://pubmed.ncbi.nlm.nih.gov/30814732/, https://pubmed.ncbi.nlm.nih.gov/31152140/]. Our current research will contribute to a broad mechanistic understanding of the transcriptional mechanisms operating in tumor-infiltrating immune cells, and may spark improved immunotherapies for cancer patients.
More recently, our laboratory has focused on the role of epigenetic regulators in gene expression. In 2009, our laboratory described the enzymatic activities of the three mammalian TET (Ten-Eleven-Translocation) proteins, TET1, TET2 and TET3 [https://pubmed.ncbi.nlm.nih.gov/19372391/]. TET proteins are dioxygenases that oxidize 5-methylcytosine (5mc) to 5-hydroxymethylcytosine (5hmC) and further oxidized products (5fC, 5caC). TET proteins regulate gene expression and enhancer activity, and play a central role in cell lineage specification, embryonic development, neuronal function and cancer [https://pubmed.ncbi.nlm.nih.gov/31965999/]. There is a notable association between TET loss of function and cancer. TET2 loss-of-function mutations are frequently observed in myeloid and lymphoid malignancies, but many other cancers – solid cancers as well as cancers of the blood – can be identified by the fact that they display low 5hmC levels even in the absence of any mutations in TET2 or the other TET genes [https://pubmed.ncbi.nlm.nih.gov/21057493/]. Indeed, diverse metabolic aberrations, found in many solid cancers, lead to profound losses of TET function and likely contribute to oncogenesis [https://pubmed.ncbi.nlm.nih.gov/25132561/, https://pubmed.ncbi.nlm.nih.gov/26595486/, https://pubmed.ncbi.nlm.nih.gov/31467060/].
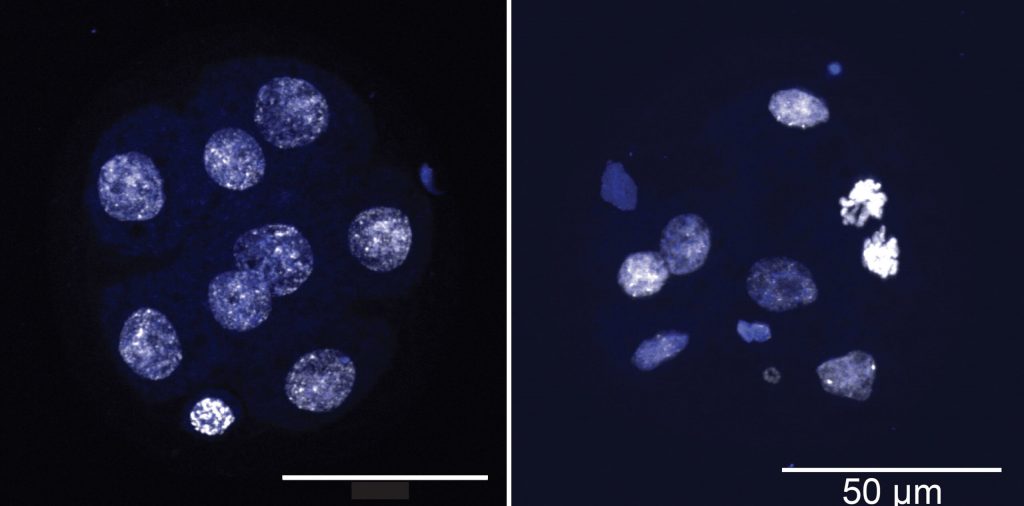
Using inducible mouse models developed by our laboratory, we have shown that deletion of two or more TET proteins leads to aggressive blood malignancies, characterized by increased levels of DNA damage [https://pubmed.ncbi.nlm.nih.gov/26607761/]. Our current efforts aim to understand the role of TET proteins in maintaining genome integrity. TET loss-of-function is associated with increased DNA methylation, hence the tumor suppressive role of TET proteins has been assumed to involve their ability to maintain DNA in a demethylated state. However, our more recent work has shown that loss of TET proteins also results in a paradoxical loss of DNA methylation in heterochromatin, due at least in part to genome-wide relocalisation of DNA methyltransferases [https://pubmed.ncbi.nlm.nih.gov/31371502/]. Widespread losses of DNA methylation in heterochromatin are also observed in aged and senescent cells. We are currently using models of cancer as well as aging and senescence to investigate the relation between decreased DNA methylation in heterochromatin, reactivation of transposable elements, and DNA damage.